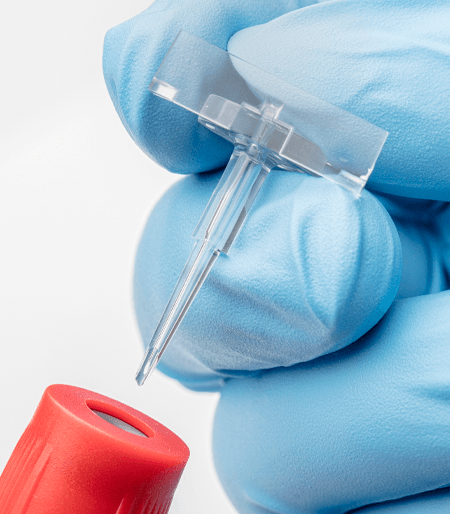
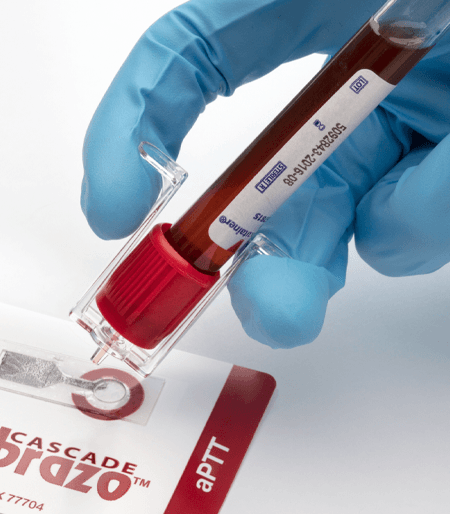
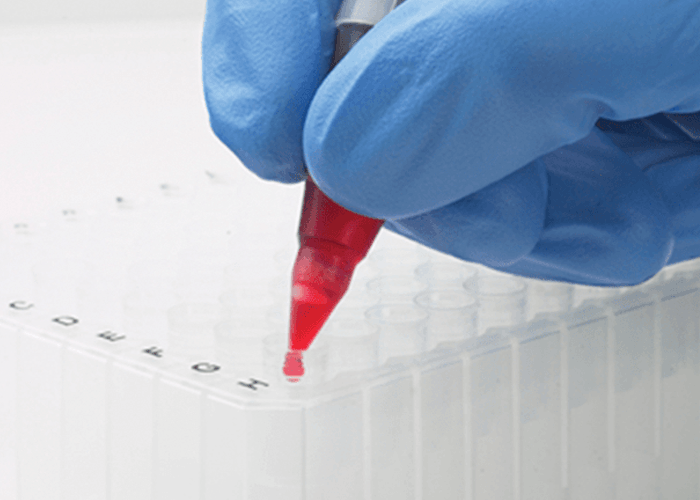
Tip Top™ Blood Tube Dispenser
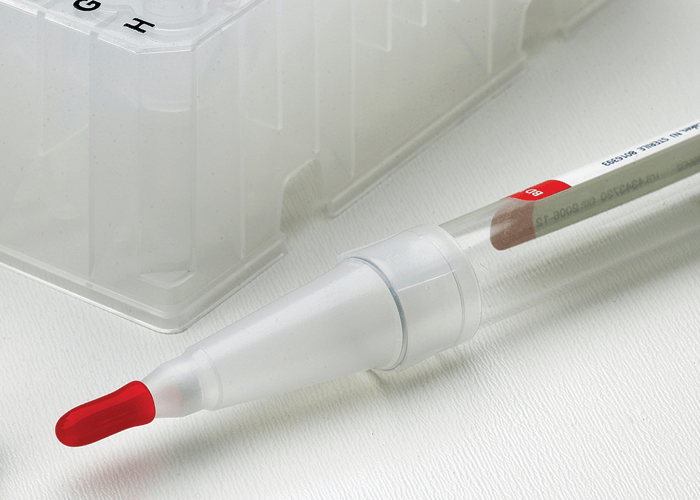
Tip Top® is a convenient alternative to pipetting from an open blood tube, just tilt the tube and squeeze to dispense one drop. And Tip Top® is safe. You don't need to pipet out of the tube so spillage is reduced along with potential exposure to pathogens. Just carefully remove the stopper and replace with Tip Top®.
Tip Top® also helps prevent evaporation, with an available flexible cap to cover the Tip Top® opening so you can store samples in the refrigerator.
Safe Blood Dispensing
The Tip Top® dispenser cap can be used with almost any liquid. Made from chemically resistant polyethylene they can dispense serum, reagents, plasma, whole blood and CSF. The integral porous polyethylene filter efficiently separates serum and can also be used as a reagent blender.
Tip Top® dispenser caps are available in resealable medical grade packaging.
The Tip Top® is a disposable cone shaped cap available in two sizes that fit all standard 13 or 16mm blood collection tubes. A small opening allows you to dispense a sample without removing the Tip Top® cap so you can prepare smears on slides or dispense into reagent wells or microcentrifuge tubes safely. Inside the tip of the Tip Top® is an integral porous filter that removes fibrin from the blood sample allowing for a perfect slide smear.
Tip Top® caps are easy to use. Making the tedious job of making blood smears simple and greatly improving lab safety.
Available in resealable non-sterile bulk bags.
| Endotoxin Free (Non pyrogenic) Product samples are exposed to endotoxin-free water and the resulting extraction fluid is tested for contamination using the kinetic turbidimetric Limulus Amoebocyte Lysate (LAL) assay protocol and USP guidelines. All products tested must display less than 0.05 EU/ml to be certified free of endotoxin. |
|
| Nuclease Free (RNase/DNase) Product samples are exposed to nuclease-free water and the resulting extraction fluid is tested for nuclease activity on commercially available 7.5 kb Poly(A) tailed RNA (1µg) and HindIII-digested DNA (1µg) with a one hour 37°C incubation in appropriate buffers. Results are visualized on an agarose gel with appropriate positive and negative controls. Extraction fluid samples must show no degradation of the nucleic acids by the extraction fluid has occurred for the product to be certified as RNase-free and DNase-free |
|
| Adenosine Triphosphate (ATP) Product sample surfaces are tested for the presence of adenosine triphosphate (ATP) using a controlled bioluminescence reaction to detect contamination. Luminescence data is compared to results generated by ATP-free surfaces and surfaces with known amounts of ATP as a positive control. The relative light units result must indicate less than 2 X 10-12 mg/µl of ATP for the product to be certified as ATP free. |
| Bovine Spongiform Encephalopathy-Transmissible Spongiform Encephalopathy These products contain resins that are processed under conditions proven to exceed the European Union standard as listed in the 22nd Commission Directive EC 98/16/EC of March 5th, 1998 as annexed to council Directive EC 76/768/EEC and further Amendment 419 Annex II of 12 June 2001. |
|
| Medical Grade (USP) U.S Pharmacoepia Methods and Guidelines (U.S.P Class VI) are used if applicable. We only use medical grade resins and pre-test all resins for contaminants prior to use. Resins are compliant with FDA CFR title 21-177.1520, 178.3295, 178.3297. |
|
| California Prop 65 No Labcon manufactured disposables contain any of the “listed chemicals” as referenced in the California Safe Drinking Water and Toxic Enforcement Act of 1986, (Prop 65) as revised May 25, 2018. |
|
| Phthalates & Oleamide All our resins are medical grade and are certified free of Bisphenol A (BPA), Oleamide, DiHEMDA, and Phthalates. |
|
| Substances of Very High Concern & REACH All Labcon products are compliant with RoHS Directive 2002/95/EC/-2011/65-2015/863, are free of Substances of Very High Concern (SVHC), and are EU REACH Regulation (EC) No 1907/2006 compliant. |
|
| Origin These products are Made in USA and all components meet the requirements for US origin under the NAFTA agreement. |
| U.S. FDA Registered Labcon is a U.S. FDA registered medical device manufacturer. Our facility is registered by the U.S. government to comply with CFR21 GMP regulations for manufacturing medical devices. |
|
| CE Compliant As applicable Labcon products meet the requirements for CE marking. |
|
| ISO 9001 Quality Registration Labcon has been registered to the ISO 9001 quality management system standar since 1997 and maintains registration to ISO 9001:2015. |
| Material | 100% Polyethylene |
| Grade | Medical |
| Rating | USP Class VI |
| Colors | Natural (White) |
| Size | 13mm & 16mm |
| Approved Use | Medical, Clinical |
| Lot Expiration | 6 years |