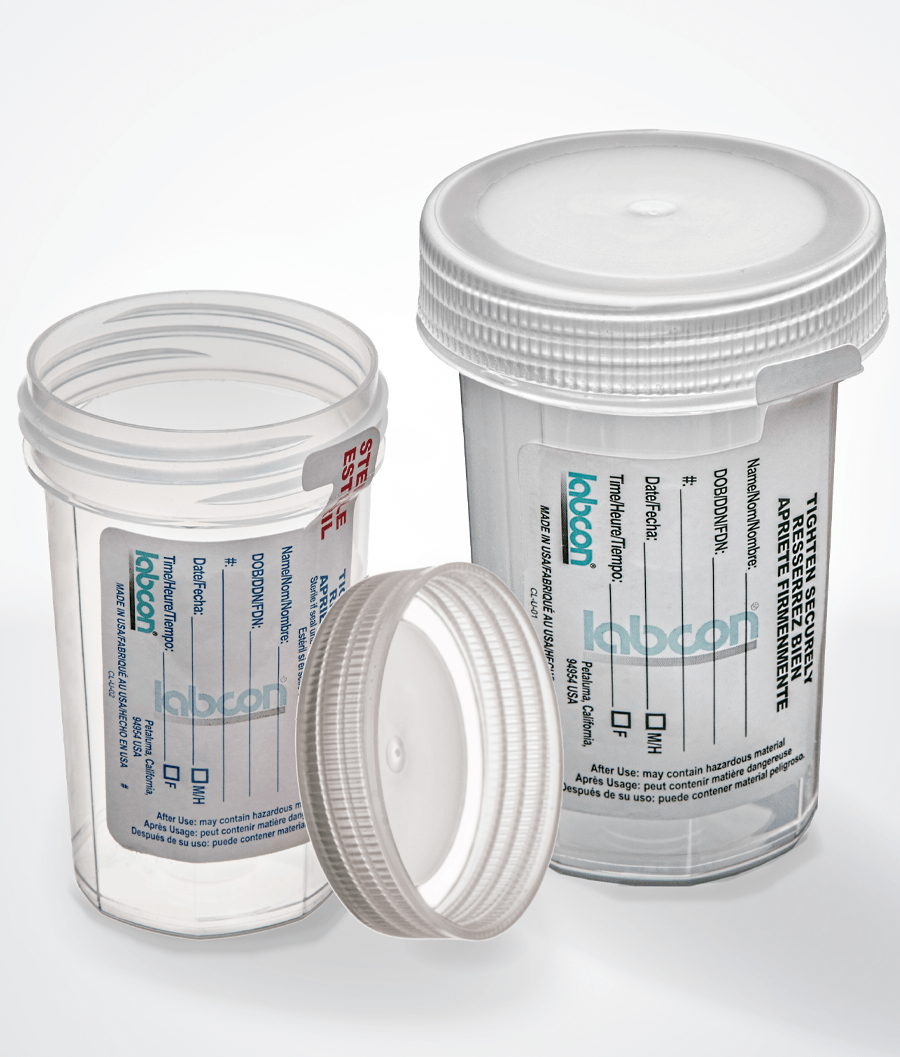
UrineSafe™ Urine Collection
UrineSafe™ urine collection and transport cups are available in both 90mL or 120mL volumes with the option of sterile (SAL 10-6) or non-sterile cups with safety labels.
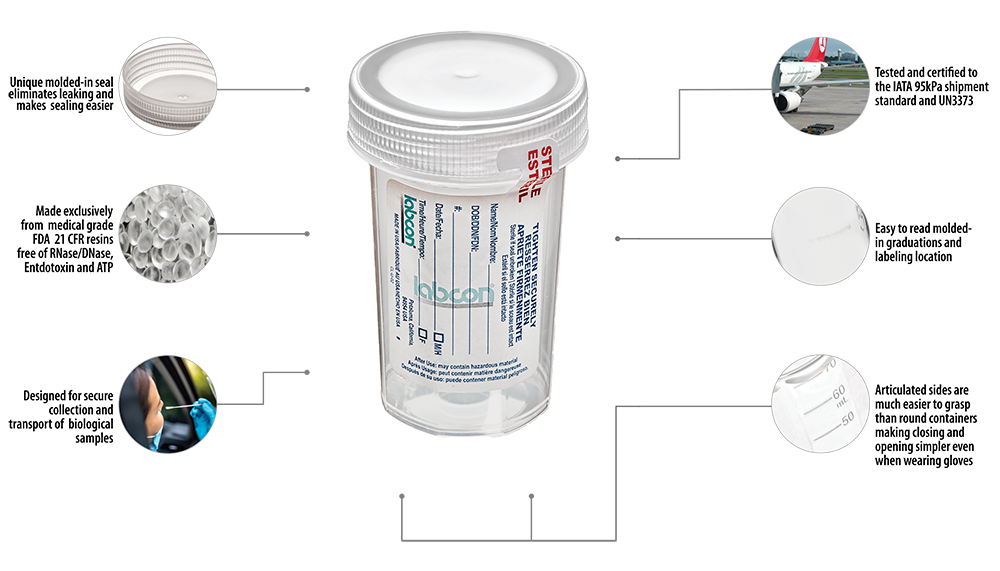
These cups include a unique cap with a molded-in elastomeric seal that virtually eliminates leakage. This ensures your sample can be moved throughout the lab safe and dry. And these cups have a seal that can be used with remote sample transport.
![]()


Safer Transport
Unlike other urine sample cups, these cups are incredibly durable, preventing the potential for leakage if dropped. These collection cups are perfect for your critical sampling needs when you can't rely on low quality cups.
Unlike commodity grade collection cups these premium cups have been tested and are certified to meet or exceed IATA requirements for safe air transport.
UrineSafe™ collection cups are available in bulk packaging and individually wrapped medical grade sterile SAL 10-6for your most critical appplications.
UrineSafe™ collection cups are made of durable medical grade resin. The caps are an exclusive design with a deep molded in place elastomeric sealing ring. The body of the cups have flat surfaces so the cups are much easier to hold with all hand sizes. Both the cap and cup are autoclavable in a steam autoclave.
Our UrineSafe™ collection cups have been tested to 95kPa to ensure you can use them for transport by ground and air shipping. And they fit most secondary safety bags and boxes.
Available in sterile individually wrapped and in non-sterile bulk bags.
| Endotoxin Free (Non pyrogenic) Product samples are exposed to endotoxin-free water and the resulting extraction fluid is tested for contamination using the kinetic turbidimetric Limulus Amoebocyte Lysate (LAL) assay protocol and USP guidelines. All products tested must display less than 0.05 EU/ml to be certified free of endotoxin. |
|
| Nuclease Free (RNase/DNase) Product samples are exposed to nuclease-free water and the resulting extraction fluid is tested for nuclease activity on commercially available 7.5 kb Poly(A) tailed RNA (1µg) and HindIII-digested DNA (1µg) with a one hour 37°C incubation in appropriate buffers. Results are visualized on an agarose gel with appropriate positive and negative controls. Extraction fluid samples must show no degradation of the nucleic acids by the extraction fluid has occurred for the product to be certified as RNase-free and DNase-free |
|
| Adenosine Triphosphate (ATP) Product sample surfaces are tested for the presence of adenosine triphosphate (ATP) using a controlled bioluminescence reaction to detect contamination. Luminescence data is compared to results generated by ATP-free surfaces and surfaces with known amounts of ATP as a positive control. The relative light units result must indicate less than 2 X 10-12 mg/µl of ATP for the product to be certified as ATP free. |
| Bovine Spongiform Encephalopathy-Transmissible Spongiform Encephalopathy These products contain resins that are processed under conditions proven to exceed the European Union standard as listed in the 22nd Commission Directive EC 98/16/EC of March 5th, 1998 as annexed to council Directive EC 76/768/EEC and further Amendment 419 Annex II of 12 June 2001. |
|
| Medical Grade (USP) U.S Pharmacoepia Methods and Guidelines (U.S.P Class VI) are used if applicable. We only use medical grade resins and pre-test all resins for contaminants prior to use. Resins are compliant with FDA CFR title 21-177.1520, 178.3295, 178.3297. |
|
| California Prop 65 No Labcon manufactured disposables contain any of the “listed chemicals” as referenced in the California Safe Drinking Water and Toxic Enforcement Act of 1986, (Prop 65) as revised May 25, 2018. |
|
| Phthalates & Oleamide All our resins are medical grade and are certified free of Bisphenol A (BPA), Oleamide, DiHEMDA, and Phthalates. |
|
| Substances of Very High Concern & REACH All Labcon products are compliant with RoHS Directive 2002/95/EC/-2011/65-2015/863, are free of Substances of Very High Concern (SVHC), and are EU REACH Regulation (EC) No 1907/2006 compliant. |
|
| Origin These products are Made in USA and all components meet the requirements for US origin under the NAFTA agreement. |
| U.S. FDA Registered Labcon is a U.S. FDA registered medical device manufacturer. Our facility is registered by the U.S. government to comply with CFR21 GMP regulations for manufacturing medical devices. |
|
| CE Compliant As applicable Labcon products meet the requirements for CE marking under regulation 2017/746. |
|
| ISO 9001 Quality Registration Labcon has been registered to the ISO 9001 quality management system standar since 1997 and maintains registration to ISO 9001:2015. |
| Material | 100% Polypropylene |
| Grade | Medical |
| Rating | USP Class VI |
| Colors | Natural (Clear) |
| Volume | 90mL & 120mL |
| Approved Use | Medical, Clinical |
| Lot Expiration | 6 years |
| Sterile Expiration | 5 years |